Unstained bacterial cells are nearly always transparent when observed under the light microscope and hence are difficult to see. In wet preparations, due to the closeness of the refractive indices of glass, water anq bacteria, the problem is compounded.
To increase the contrast between the bacterium and its surroundings therefore, staining is employed. Most bacteria can be readily stained in solutions of certain dyes or stains. Staining to produce artificially colored microorganisms _ renders microbial cells easier to visualize microscopically. Staining can be regarded as rendering cells.especially bacteria visible and more distinct from its surroundings by the application of dyes. In other words, staining is simply the art of imparting colors to cells using coloring materials (dyes).
The purpose of staining is to add contrasts to the image, to enable the scientist identify the particular chemical components of interest and to locate particular cells, tissues or organelles. Stains are colored salts which readily dissolve in water to form positive ions (cations) and negative ions (anions). If the color component of a stain is carried by an anion, the stain is an acid stain. The anions of the acid stains are attracted by the positively charged (acidophilic) parts of the cell, such as the cytoplasm. Acid stains are usually colored salts of sodium, calcium, ammonium or potassium.
Examples of acid stains are methyl red, acid fuchsin, congo red, Indian ink, nigrosin, orange G, fluorescein etc. If the colored component of a stain is carried by a cation, the stain is known as a basic stain.
Basic dyes have affinity for acidic (negatively charged) parts of a cell, e.g. nucleus. Basic stains include basic fuchsin, crystal violet, gentian violet, methylene blue, malachite green and safranin. Neutral dyes contain color components carried by both the positive and the negative charges which can respectively stain the acid and the basic components of a cell. Examples include eosin, giemsa, Wright’s solution and Leishman’s stain.
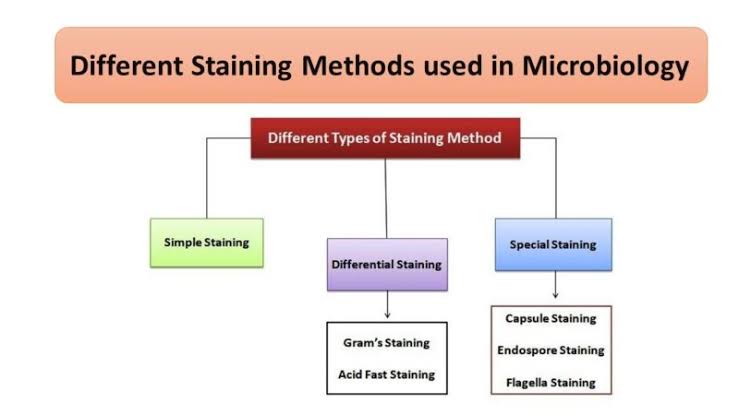
TYPES OF STAINS
1. Simple stains:
When only one dye is used and no particular structure is revealed, the staining technique is referred to as simple. Examples of simple stains include methylene blue, dilute carbol fuchsin.
2. Differential stains:
Differential stains are used to illustrate chemical differences existing within the cell or its surface. Two or more dyes may be used in the technique. Differential stain technique distinguishes two different kinds of organisms, e.g. Gram stain technique, Ziehl-Neelsen staining technique.
3. Special stains:
These are used to reveal special structures like bacterial capsules, flagella and spore$, e.g. Indian ink, Malachite green, Nigrosin, Congo red “ etc.
STAINING TECHNIQUES:
1. GRAM’S STAIN
The gram staining technique was introduced by Christian Gram, a Danish physician in 1884. It is the staining Method most commonly used in bacteriology since it differentiates nearly all bacteria into two main groups -Gram-positive and Gram negative, a differentiation which is of great value in the identification of a bacterium.
PRINCIPLE OF GRAM STAINING
When stained with crystal violet and Lugol’s iodine, all bacteria stain alike, a deep blue or violet color. The differences in composition between gram positive and gram negative cell wall (which contain thick peptidoglycan with numerous techoic acid crosslinkages) and gram negative cell walls (which consists of a thinner layer of peptidoglycan and an outer membrane) accounts for the gram staining differences between these two major bacterial groups.
Gram positive organisms resist alcohol decolorization and appear deep-blue. On the other hand, gram negative organisms are decolorized and appear red with safranin counter stain.
NOTE: The crucial step in Gram staining is that of decolorization with acetone. If this is excessive, Gram positive bacteria will be decolorized and appear Gram negative. Conversely, if decolorization is insufficient, Gram negative bacteria will not be decolorized and will appear gram positive. In either of the cases, the identity of the bacterium is lost.
2. ZIEHL-NEELSEN STAIN (ZN) FOR ACID FAST BACILLI
The Ziehl-Neelsen (ZN) staining technique is a differential staining valuable in distinguishing the acid fast bacilli (AFB). Acid fastness is a characteristic property of the Mycobacteria and some Actinomycetes. The high lipid content of these bacteria prevents them from staining readily with dilute solutions of dyes such as employed in the gram f stain.
However, they do stain when heated with strong (concentrated) dye solutions and are resistant to decolorization by acid and alcohol (i.e they are acid fast). Common examples of AFB include Mycobacterium tuberculosis which causes tuberculosis, M. leprae (leprosy). M. bovis -from unpasteurized milk (tuberculosis), M. smegmatis (non-pathogenic), Norcadia asteroids (pulmonary disease), and N. brasiliensis (mycetoma).
3. THE SPORE STAIN (SPECIAL STAINING TECHNIQUE)
Spores are produced within the cell of bacteria and are called endospores. Endospores are oval or spherical. They are commonly produced by the genera Bacillus, Clostridium, Sporosarcina, Sporolactobacillus, Desulformaculum, Oscillospira, etc. Endospores are generally formed in response to adverse environmental conditions such as depletion or shortage of carbon, nitrogen and/or phosphorus or accumulation of waste products.
Sporulation is a means of survival under unfavorable conditions. Spores are of economic importance in food/canning industries, medicine, agriculture, biotechnology (bioterrorism), and biomedical sciences. They are drought and heat resistant.
The location, shape, size and type of spore, help in the identification of species of spore formers. Based on the location of spore within a cell, a spore maybe central spore if it is formed at the centre as in Bacillus cereus, terminal, if it is formed at the end e.g. Clostridium tetani (drum stick appearance) and subterminal, if it is formed near the end of a cell, e.g Clostridium subterminale.
PRINCIPLE OF THE SPORE STAIN
Spores are not readily stained by cold dyes. In the following staining method introduced by Schaeffer and Fulton in 1933, the organisms (including their spores) are first stained a pale green colour with hot malachite green solution. Subsequent application of cold safranin superimposes a brownish red color on the vegetative cells. This contrasts well with the: – pale green color of the spores (which are not penetrated by the cold red dye).
4. DEMONSTRATION OF CAPSULES
Some bacteria secrete a polysaccharide substance which envelops the individual cell. This viscous substance forming a covering layer or envelop around the cell wall is termed capsule. Capsule increases the virulence of bacteria that possess them.
Three types of capsules are known as; Microcapsule (if it is too thin to be seen by light microscopy), _ macrocapsule (visualized by light microscopy) and slime (if it is abundant that many cells are embedded in a common / matrix).
PRINCIPLE OF DEMONSTRATION OF CAPSULES
Bacterial cells are mixed with Indian ink or Nigrosin dye and spread out in a thin film on a slide. After air drying, the ‘cells appear as lighter bodies in the midst of a blue-black background because ink particles cannot penetrate the cells or its capsule. The extent of the light region is determined by the size of the capsule and the cell itself.
QTHER DIFFERNTIAL STAINS:
1. Flagella Staining
2. Staining Of Intracellular Lipids
3. Deoxyribonucleic Acid Stain (Rainbow’s Staining)
4. Volutin Granules Stain
5. Cell Wall Stain (Hales Method)