Measurements are very important in scientific work. Reagents are prepared by dissolving specified quantities in the specified solvent. The specified quantities to be dissolved are obtained by weighing out.
The accuracy of measurements enables the scientist to obtain the desired concentration of.the reagent for the work he intends to do with it. Some Experiments are only evaluated by taking measurements, A typical example is the agar disk diffusion technique of antibiotic assay. In this experiment the results are taken by measuring the inhibition zone diameter (1ZD) in millimeters(mm) and comparing it with a standard. In other experiments the measurement of the optical density is the required results for further analysis. This can easily be done with a spectrophotometer.
Measurements in the laboratories involving determination of weights and lengths, Timing of experiments,measurement of temperature and temperature control.
Measurement of weights: Weights are measured or determined in laboratories using balances. Some balances are general purpose laboratory balances with a wide range of capacities and excellent value in precision weighing.
Mechanical and electronic balances are available in most laboratories. An example of a mechanical balance is the triple beam balance (Ohaus). Electronic balances with digital read outs are preferred to mechanical balances. These balances are easy to read. Moreso, their self taring feature isan added advantage. This means that the mass of the
weighing boat or container can be subtracted automatically before weighing an object. Common electronic balances offers accuracy down to Img over the range 1mg to 160g. This range is very suitable for most biological applications.
Measuring Time
Many experiments in microbiology need to be timed carefully. Some must be read within a particular time range.In laboratories, stop watches are used to achieve this timing.
Measuring And Controlling Temperature
Storage of samples and specimens often involve regulating the temperature of storage. Heating is also a
means of drying specimens or glassware in laboratories. Ovens and drying cabinets’ are used for drying specimen and glasswares. Ovens and drying cabinets are usually thermostatically controlled and care should be taken to ensure that the temperature control knob is set at the appropriate temperature. Storing chemicals and stock solutions that would either breakdown or become contaminated at room temperature is common practice in laboratories. Refrigerators are used at temperature of 4°c and freezers at temperature of about -15°C.
MEASUREMENT OF SIZE OF BACTERIAL CELLS
Materials:
Note:
Stage micrometer, micrometer eye piece.
The micrometer eye piece is a glass scale divided intotenths or twentieths of a millimeter. The stage micrometer isdivided into hundredths of a millimeter ie. 0.01mm.
Calibration of Ocular Micrometer
Procedure
1. Mount the micrometer eyepiece in the focal plane of the eyepiece. When properly mounted it is seen in the field of view.
2. Place the stage micrometer in position on the microscope stage.
3. Focus its image which will be observed in apparent superimposition with the eyepiece scale.
4. Determine how many divisions of the eye piece scale that correspond to a definite number of division on the stage scale.
5. Calculate the measurement of an eyepiece division in microns.
Note: it is important that the objective, ocular and draw tube length be recorded for each calibration.
Measurement of Size of Bacterial Cell.
1. Prepare a suspension of the bacterium
2. Dilute enough for individual cells to be easily seen about 5 to 10 cells per drop of suspension.
3. Place a drop of the bacterial suspension on a slide, cover with a cover slip.
4. Mount the slide under the microscope and view using high power magnification.
5. Bring to focus the cells and determine the divisions on the eye piece that correspond to the diameter, length and breadth of the bacterial cells.
6. Calculate the size of the cells using the calculations already established during the calibration.
PREPARATION OF DILUTIONS
Dilution involves reducing the concentration of the original sample. In scientific work, dilution series are made in such a way that they can be easily related to the concentration of the undiluted sample. Dilution series are used in a wide range of procedures including the preparation of standard curves for analytical instruments.
In analytical microbiology and immunoassay, dilutions are very important because the undiluted sample may not give the required results.
For instance, to determine the total viable count per ml of a sample, plate counts outside the range 30-300 cfu per plate are not acceptable. Anything above this range is regarded as confluent growth and the plate is said to be overcrowded.
To get out of this problem, dilutions of samples are used to reduce the number of viable cells in the inoculum. These dilutions are made in such a way that the results obtained with them can be used to calculate the number of viable cells per millilitres of the undiluted sample.
Two methods of dilutions are very important in microbiology. These are the doubling (two-fold) and ten-fold dilutions. The doubling and ten-fold dilutions are typical examples of logarithmic dilution series.
Dilutions are made using suitable diluents. Examples of diluents used in Microbiology include normal saline, phosphate buffered saline (PBS), 0.1% peptone water and sterile water. It is important to note that other diluents are better than sterile water. See appendix for more details.
Doubling Dilutions:
This is a dilution made in such a way that the concentration of the resultant mixture is half that of the previous one, It is a two fold step interval (Log 2) dilution series.
This can be easily done by measuring out a quantity of the undiluted sample into a tube. Then an equivalent volume of diluent is added into the tube and mixed thoroughly. If this is repeated the concentrations obtained will be 1/2, 1/4, 1/8, 1/16, 1/32 etc and these dilutions will be two, four, eight, sixteen, thirty-two fold etc.
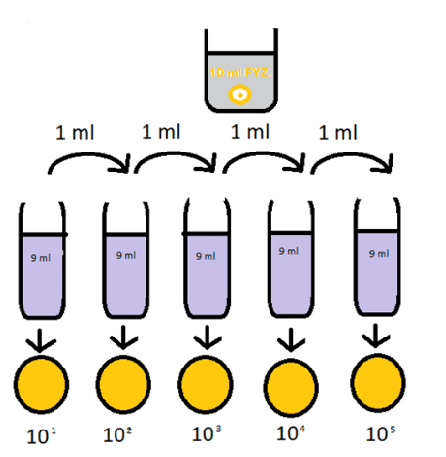
Ten Fold Dilutions (Decimal Dilutions):
Ten-fold dilutions are made in such a way that each concentration is one tenth that of the previous one. It is ten fold step interval (Log10) dilution series. This is done by first measuring out a quantity of the undiluted sample into a tube.
Then nine parts of the diluents is put into another tube. Subsequent transfer of one part of the undiluted sample into nine parts of the diluents when mixed thoroughly gives a ten fold dilution. If this is repeated, the concentration obtained will be 1/10, 1/100, 1/1000, 1/10000, 1/100,000 etc (i.e 10-1, 10-2, 103, 10-4, 10-5).