The development of microorganism on culture media is dependent upon a number of very important factors.
1. The proper nutrients must be available
2. Oxygen or gases must be available as required
3. A certain degree of moisture is necessary
4. The medium must be of the proper reaction
5. Proper temperature relations must prevail
6. The medium must be sterile
7. Contamination must be prevented.
A satisfactory microbiological culture medium must contain available sources of hydrogen donors and acceptors, carbon, nitrogen, sulphur, phosphorus, inorganic salts and in certain cases, vitamins or other growth promoting substances.
These were originally supplied in the form of the meat infusions which were and still in certain cases, widely used in culture media, beef or yeast extract frequently replace meat infusions, but the preparation of these substances subject them to the loss of their heat labile nutritive factors in much the Same way as infusions are affected. The addition of peptone provides a readily available source of nitrogen and carbon.
Peptone is used in culture media to supply an available form of nitrogen since native proteins are capable of utilizing the amino acid and other simpler nitrogenous compounds present in peptone. Continued investigation in our laboratories indicate that for the isolation and propagation of many organisms, the complicated form of media can be replaced by simpler media.
Certain bacteria require addition of other nutrients such as serum blood, or ascites to the culture medium, upon which they are to be propagated. Carbohydrates may also be desirable at times, and certain salts such as those of calcium, manganese, Magnesium, sodium, and potassium seem to be required. Dyes may be added to media as indicators of metabolic activity or because of their selective inhibitory power.
Growth promoting substances of a vitamin-like nature are essential or assist greatly in the development of certain types of bacteria.
The consistency of a liquid medium may be modified by the addition of agar, gelatin or albumin in order to change it into a solid or semisolid state. Solid media which were originally devised for the isolation of organisms in pure culture, are now universally used for almost all general cultural work. The semi – solid media are used chiefly for carrying stock cultures or for propagating the anaerobes.
Most bacteria are capable of growth under ordinary conditions or oxygen tension. Certain types, however, are capable of deriving their oxygen from various substrates. The aerobic organisms require the free admission of air, while the anaerobes grow only in the exclusion of atmospheric oxygen.
Between these two groups are the microaerophiles which develop best under partial anaerobic conditions and the facultative anaerobes which develop over a wide pH range. Anaerobic conditions for growth of microorganisms are obtained in a number of ways:
- Addition of small amount of agar to liquid mediaAddition of fre h tissue to the medium
(c) Culturing in the presence of aerobic organisms
(d) Addition of a reducing substance to the medium.
(e Displacement of the air by carbon – dioxide
(f) Absorption of the oxygen by chemicals.
(g) Removal of oxygen by direct oxidation of readily oxidizable substances such as burning a candle, heating of palladiumized asbestos, copper, hydrogen, phosphorus or other readily oxidizable metals.
(h) Incubation in the presence of germinating grain or pieces of potato.
(i) Inoculation into the deeper layers of solid media, or under a layer of oil in liquid media. (j) Combinations of these methods.
Proper moisture conditions must prevail in the culture media employed for the propagation of microorganisms. A moist medium and a moist atmosphere are necessary for the continued luxuriant growth of the vegetative cells. Incubators, if not humidity controlled, should have open containers filled with water at all times to provide sufficient moisture for growth and prevent drying of media. Growth of most microorganisms is obtained in the absence of light. Sunlight is to be avoided unless it is essential to growth as in photosynthetic bacteria.
The pH of the culture medium, expressing it’s hydrogen ion concentration, is extremely important for thé growth of mucroorganisms. The majority of the microorganisms prefer culture media which are approximately neutral, while othera may require a medium which fe distinctly acid.
The pH of the culture medium is determined by colorimetric or electrometers measurement of its hydrogen ion concentration. It should be noted that addition of acid or alkali which are insufficient to prevent the growth of bacteria in a medium may inhibit or prevent them from proceeding with the normal functions of their metabolic processes.
The usual range of temperature suitable for the growth of mesophilic microorganisms lies between 15 – 43°C. Psychrophilic microorganisms have, however, been known to develop at 0°C and others, such as the thermophilic soil organisms, may grow at 80°C. The pathogenic organisms in general are limited by a comparatively narrow range of temperature around 37°C while the saprophytes usually have a much broader range.
All organisms exhibit three cardinal points in their thermic relations:
- A minimum below which development ceases.
- An optimum at which growth is luxuriant.
- A maximum above which growth ceases and death occurs.
TECHNIQUES FOR OBTAINING PURE CULTURES
The fundamental principles of pure culture isolation and propagation still constitute the foundation of microbiological practice and research.
However, many of the problems associated with direct detection from clinical samples are absent when pure cultures of the organism are available.
Culture can generally be defined as a growth of particular type (s) of microorganism on or within a solid medium, or liquid medium, formed as a result of the prior inoculation, incubation of that medium. Growth on a solid medium may be present as a continuous layer or film (confluent growth) or as discrete (individual) colonies depending on the method of inoculation, such as lawn plate and streaking.
This normally lead to contaminated culture or mixed culture which is one containing two or more species or strains of organisms. The first step in culturing an organism is to make the primary culture which is the process of preparing a culture by incubating a medium that has been inoculated with (or from) a specimen.
Several subcultures are then made to obtain a pure culture which is one comprising of organisms which are all of the same species or strain. It is also referred to as axenic culture.
We have different methods of culture such as the shake culture formerly used for the culture and isolation of anaerobes. The inoculum is dispersed, by shaking in a molten agar medium (about 48°C) contained in a long glass test tube; the medium may contain a reducing agent.
We also have the slant culture which is one grown on a solid medium (usually agar) which has been allowed to set in a test tube or bottle placed at an angle to the horizontal. There is also the stab culture which is one produced by deep inoculation of a solid medium (e.g agar or gelatin) with a straight wire; the wire is plunged vertically into the medium so that the inoculum (on the tip of the wire) is distributed along the length of the stab.
In order to study the properties of a given organism, it is necessary to handle it in pure culture free of all other types of organisms. To do this a single cell must be isolated from all other cells and cultivated in such a manner that its collective progeny also remain isolated. Several methods are available:
1. PLATING TECHNIQUE:
Cells in or on a gelled medium are immobilized unlike cells in a liquid medium, Therefore, if few cells are placed in or on a gelled medium, each cell will grow into an isolated colony.
The ideal gelling a ent for most microbiological media is agar, which is an acidic polysaccharide extracted from certain red algae. A 15 2% suspension in water dissolves at 100°C, forming a clear solution that gels at 45°C.
Thus, a sterile agar solution can be cooled=50°G, bacteria or other microbial cells added, and then the solution quickly cooled below 45°C to form a gel (although most microbial cells are killed at 50°C, the time course of the killing process is sufficiently slow at this temperature to permit this procedure. Once gelled, agar will not again liquefy until it is heated above 80°C, so that any temperature suitable for the incubation of a microbial culture can subsequently be used.
POUR PLATE METHOD
In the pour plate method, a suspension of cells is mixed with melted agar at 50°C and poured into a petri dish. When the agar solidifies, the cells are immobilized in the agar and grow into colonies. If the cell suspension was sufficiently diluted, the colonies will be well separated, 90 that each has a high probability of being derived from & single cell.
To make certain of this, however it is necessary to pick a colony of the desired type, suspend it in water, and replate. Repeating this procedure several times ensures that a pure culture will be obtained.
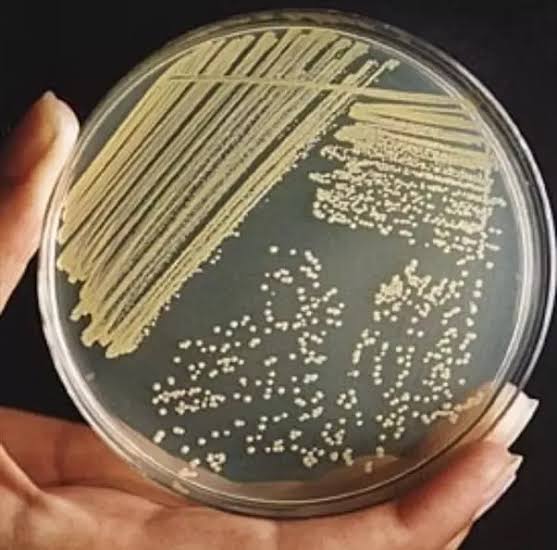
3. STREAKING TECHNIQUE
Alternatively, the original suspension can be streaked on an agar plate with a wire loop. As the streaking continues fewer and fewer cells are left on the loop, and finally the loop may deposit single cells on the agar.
The plate is incubated, and any well isolated colony is then removed resuspended in water, and again streaked on agar. This method is just as reliable as and much faster than the pour plate method.
2. DILUTION TECHNIQUE:
A much less reliable method is that of extinction dilution. The suspension is serially diluted, and samples of a particular dilution exhibit growth. It is presumed that some of these cultures started from single cells.
This method is not used unless plating is for some reason impossible. An undesirable feature of this method is that it can only be used to isolate the predominant type of organisms in a mixed population.
ASEPTIC TECHNIQUE:
Aseptic technique is using sterilised equipment and solutions and preventing their contamination while in use. Bacteria and fungal spores are abundant in most environments, including laboratories.
The microbiologist uses a range of special techniques and apparatus which are designed to prevent contamination of nutrient media. Special laboratories are needed for routine microbiological work. These will have easy to clean surfaces and special enclosed benches which receive filtered sterile air. ”
SPREAD PLATE CULTURE
1. Prepare a serial dilution of the sample up to 10-⁵.
2. Transfer a known, small volume (up to 0.5cm’) of cell suspension to the surface of the nutrient agar in a petri dish. A tap on the end of the pipette helps to control delivery.
3. Clean the spreader with alcohol and allow excess alcohol to drain from it. Ignite the alcohol by passing the spreader through a bunsen flame. Do not leave in the bunsen flame or it may crack. (Alcohol burns at much lower temperature than that of the bunsen flame). After the flame has disappeared, allow to cool for about 10 seconds.
4. Spread the liquid over the surface of the agar using the spreader. Rotate the plate at the same time to ensure an even courage. Label the base of the plate and incubate. Store the spreader in the beaker after use.
STAB CULTURE:
This is used for anaerobic organisms or those that thrive in conditions of low oxygen concentration, namely microaerophiles. An nutrient agar medium in a test tube is normally used.
The greater depth of agar, and the small surface area in the tube compared with a dish, means less oxygen can diffuse – into the agar. Inoculation is done with a straight wire (not loop). A sample (liquid or solid) is collected at the tip of the wire which is then stabbed vertically through the medium. The culture grows out from the stab line.
Procedure
1. Collect a portion of the sample with a wireloop.
2. Stab vertically through the medium
3 Incubate at 7c for 24h
4. Observe for growth from the stab line
INOCULATING A LIQUID MEDIUM
If the cells to be inoculated are in a liquid, example water, milk or a broth, a sterile wire loop is usec transfer a sample to the medium, which is often in a t tube. The wire loop is simply agitated gently inside | medium. Remember to flame the necks of bottles if caps or cotton wool plugs are removed. If the cells to be inoculated are in or on a solid medium, such as soil or nutrient agar, a wire loop may also be used for transfer to the liquid medium. It can be rubbed on the inside surface of the vessel containing the liquid medium and then can be tapped afterwards to help mix the culture.
IDENTIFICATION OF BACTERIA
Computation is now an essential tool for the construction, evaluation, and application of these data for the identification of unknown strains of medical, industrial, ecological or scientific importance.
The ever increasing rates of isolation of novel strains and their generation by manipulation in vitro require the continued development of rapid and objective computerized systems for identification.
PRINCIPLES OF BACTERIAL IDENTIFICATION
The taxonomy of any group of organisms is based on three sequential stages: Classification, nomenclature, and identification. The first two stages are the prime concern of professional taxonomist but the end product of their studies should bear the identification system that is of practical value to other worked. Therefore, an identification system is clearly dependent on the accuracy and data content of classification schemes and the predictive value of the name assigned to the defined taxa.
The ideal identification system should contain the minimum number of features required for a _ correct diagnosis which is predictive of the other characters of the taxon identified. However, the minimum number of characters required is dependent on both the practical objectives of the exercise and the clarity of the taxa defined in classification. Thus many enterobacteria can be identified using relatively few physiological and biochemical tests.
The numerous serotypes of Salmonella are recognized by their reactions to specific antisera, and the accurate identification of Streptomyces species requires determination of up to 50 diverse characters. However, many new “species” of streptomycetes have been proposed ‘solely on their ability to produce a novel metabolite.
SOURCES OF DATA FOR BACTERIAL IDENTIFICATION
The sources of information may be categorized as follows:
1. . Morphology and physiology: e.g, gross and fine structure, motility, nutrition, and enzyme production)
2. Cell components (e.g, wall composition, lipids, whole cell pyrolysis)
4. Proteins (e.g sequences, serology, phage typing)
5. Nucleic acids (e.g DNA/DNA hybridization, nucleic acid probes)
Traditionally, microscopy has provided the most rapid indication of whether or not a patient is suffering from an infection and ite importance is undiminished.
COLONY /CULTURAL CHARACTERISTICS
Media which contain an indicator enable colonies of one organism to be visibly distinguished from those of another. For example, certain bacteria can break down (“ferment”) the sugar lactose to an acid. Ina medium such as MacConkey’s agar, which contains the pH indicator neutral red and bile salts, colonies of lactose fermenters will appear red and non-lactose fermenters colourless or pale pink.
Similarly, if Salmonella were present in a sample of gut bacteria it would form colourless colonies in MacConkey’s agar.
The morphological characteristics of pure colonies are useful in the identification of bacteria. To this end, the colonies of pure cultures growing on solid media should be examined, with special reference to their size, the pattern of their edge or margin, their surface texture, elevation, consistency and colour or pigmentation.
It has to be borne in mind that some, or even all these characteristics may change, depending on the media on which the organisms are growing.
Nevertheless, some of these characteristics are very striking that they compel considerable attention. The examination of these colonial characteristics are carried out under the following headings.
1. SIZE:
Colonies vary in size over a broad range, some of them being as small as pin heads, whereas others can grow to a diameter of 10mm.
The usefulness of using size as a distinguishing feature is however limited by the fact that size changes with changes in prevailing conditions. For example, colonies growing in conditions of overcrowding tend to be considerably smaller than those of the same organism growing on plates containing only a few colonies.
This is because of the greater competition for nutrients by organisms growing on overcrowded plates. It has to be said however that for most organisms, there is an upper limit to the size which the colonies can attain.
The exceptions to the rule are found in some genera, for example, Proteus and bacillus spread continuously during growth so that a single colony is able to cover the entire surface of the plate on which it is growing.
2. EDGE:
Edge of colonies have characteristic patterns. They may be entire or show irregularities which make them undulate, lobate, fimbriate, striated, crenated or even rhizoid.
3. ELEVATION:
Colonies may be flat, raised, convex, umbonate or papillate.
4. SURFACE:
The surface of a colony may be smooth (shining or glistening), rough (wrinkled or granular), or mucoid (slinging). Mucoid colonies are developed from encapsulated organisms and as earlier mentioned, this has implications for their pathogenicity since they are hereby protected from phagocytosis.
Some mucoid organisms however mutate to produce colonies which are rough, and some organisms can mutate and lose their ability to produce capsules. Such organisms also lose their ability to cause mfection.
5. COLOURS:
Many colonies because of the large number of cells they contain, are seen to be beige. However there are some organisms which because of their ability to produce pigments give rise to colonies which have some characteristic colours.
For example, colonies of Serratia marcescens are red, those of Staphylocuccus aureus are golden yellow when grown on nutrient agar containing 1% glycerol monoacetate. Chromobacterium violaceum give rise to purple colonies. The colonies of these organisms are coloured, because the pigments are not water soluble and are therefore retained within the colony.
Some other organisms, for example, Pseudomonas aeruginosa produce a water soluble pigment pyocyanin which diffuses into the surrounding medium and gives it a characteristic greenish tinge. This organism produces another pigment, pyovedin, which is fluorescent so that the medium around its colonies glows white or bluish-green when exposed to ultra-violet light;
6. CONSISTENCY:
Most colonies have a_ butter-like consistency. Such colonies are easy to pick up and transferred with inoculating needles, unlike other colonies which may be viscous, stringy or rubbery. Portions of such colonies are difficult to pick up because the whole colony is – usually adhered to the needle. Some other colonies are dry, brittle or powdery.